Introduction to NV Centers, Electron Spin, and ODMR
1. What is an NV Center?
An NV center (“Nitrogen-Vacancy”) is a point defect in the crystal lattice of diamond, consisting of:
- a nitrogen atom replacing a carbon atom, and
- an adjacent vacancy.
In its negatively charged form (NV⁻) it contains 6 electrons, distributed over four molecular orbitals ($a_1$, $a_1'$, $e_x$, $e_y$). Two electrons with parallel-aligned spin occupy the degenerate orbitals $e_x$ and $e_y$, resulting in a total spin S = 1.
2. What is Spin?
Spin is a fundamental quantum-mechanical property of particles, analogous to an intrinsic angular momentum. It is:
not a real rotation, but a quantized property with two possible states for the electron:
responsible for:
- the Pauli principle: no two electrons can occupy the same quantum state.
- Hund’s rule: orbitals are singly filled with parallel spins first.
In NV centers the spin value is even $S = 1$, allowing three projections: $m_s = 0, +1, -1$.
3. Energy Levels in the NV Center
The NV center has:
- a triplet ground state $|g⟩$,
- a triplet excited state $|e⟩$,
- a non-fluorescent singlet intermediate state $|s⟩$.
Through spin-spin interactions (even without a magnetic field!), the ground state is split into:
- $|g, m_s = 0⟩$ and
- $|g, m_s = ±1⟩$
The energy splitting is:
4. Optically Detected Magnetic Resonance (ODMR)
Basic Idea
- The NV center is excited with green light (532 nm).
- It fluoresces in the red range (~637–800 nm) depending on the spin state.
Fluorescence Pathways
- $|e,0⟩ \rightarrow |g,0⟩$: radiative transition → bright fluorescence
- $|e,±1⟩ \rightarrow |s⟩ \rightarrow |g,0⟩$: non-radiative → dark
Microwave Excitation
- Microwaves at 2.87 GHz induce spin flips between $|g,0⟩$ and $|g,±1⟩$.
- The electron ends up in a less fluorescent state ⇒ dip in fluorescence.
With External Magnetic Field
- A static magnetic field along the NV axis causes a Zeeman effect, further splitting $|g,+1⟩$ and $|g,-1⟩$.
- Two resonance frequencies appear ⇒ two dips in the ODMR spectrum.
5. Key Questions and Answers
▶ Why do the $m_s = ±1$ states split?
Due to zero-field splitting from spin-spin interaction in the crystal field.
▶ What is the singlet state?
A non-radiative intermediate state through which electrons from $|e,±1⟩$ relax to $|g,0⟩$ – without photon emission.
▶ How does the electron go from $|g,0⟩$ to $|g,±1⟩$?
Via targeted microwave excitation at 2.87 GHz, matching the energy splitting.
6. ODMR Signal – What Is Measured?
- The ODMR signal is the fluorescence intensity as a function of microwave frequency.
- Without microwaves: maximum fluorescence.
- At resonance: fluorescence decreases (spin is flipped out of $m_s = 0$).
- With magnetic fields: two absorption dips instead of one.
7. Why Is This Important?
- Quantum sensing: precise measurement of magnetic fields (nanotesla range).
- Quantum information: spin states serve as qubits.
- Biophysics & materials science: sensors for temperature, electric fields, pH, etc.
8. Summary Mnemonic
9. States:
1. Optical Excitation Only (532 nm, no Magnetic Field, no Microwave)
The NV center is excited with a 532 nm laser: electrons go from the ground state $|g⟩$ to the excited state $|e⟩$. There are two pathways back:
- $|e,0⟩ \rightarrow |g,0⟩$ with strong fluorescence
- $|e,±1⟩ \rightarrow$ non-radiative transition via singlet state $|s⟩ \rightarrow |g,0⟩$ ⇒ reduced fluorescence
Result: most NV centers end up in $|g,0⟩$. This is called optical pumping into the $m_s = 0$ state.
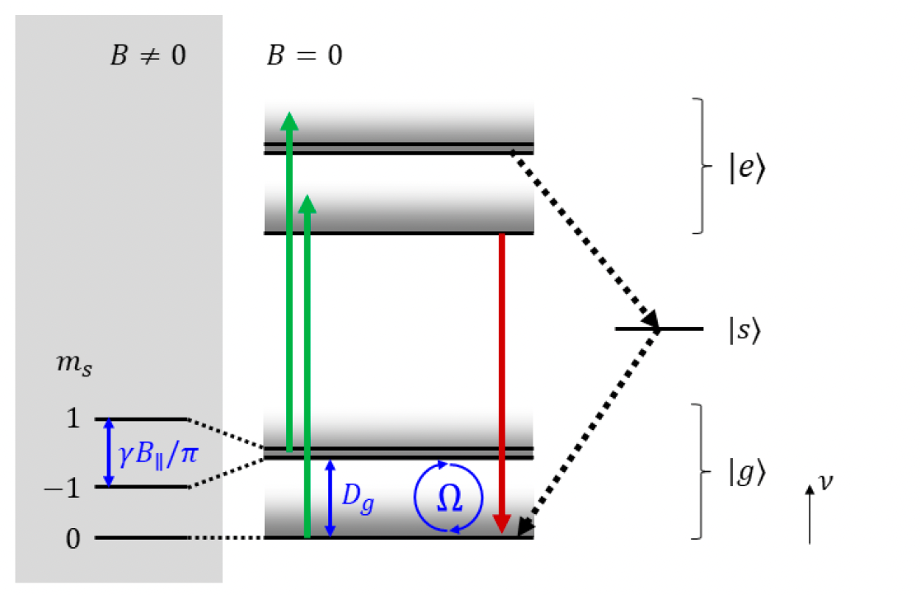
2. Optical Excitation + Microwave (2.87 GHz)
A microwave at 2.87 GHz matches the splitting between $|g,0⟩$ and $|g,±1⟩$ (zero-field splitting $D_g$). This induces a spin flip:
- Electrons are periodically swapped between $|g,0⟩ ⇄ |g,±1⟩$ (Rabi oscillations).
Consequence: more electrons end up in $|e,±1⟩$, which then relax via the singlet state ⇒ fluorescence darkens.

3. Optical Excitation + Microwave + External Magnetic Field
An external magnetic field $B∥$ along the NV axis splits the degenerate $m_s = ±1$ level (Zeeman effect). Now there are two distinct resonance frequencies: $|g,0⟩→|g,+1⟩$ $|g,0⟩→|g,-1⟩$ Sweeping the microwave frequency → two dips in fluorescence. This is the typical ODMR signal under a magnetic field.
